Due to a recent change in our pharmacy software system, the process for submitting refill requests online has now changed.
Our previous mobile app and your current login credentials will no longer work.
Please click the Patient Portal tab to begin the new process.
Thank you for your patience during this transition.
Appointment Only at Deerfield location. Click button to schedule.
Details:
- Location: Boone Drug at Deerfield, 345 Deerfield Road Boone, NC 28607
- By Appointment Only – Please check appointment link above for the available clinic dates.
- Cost – Boone Drug will attempt to file claim on patient’s pharmacy insurance, in the event the claim is not covered the patient will be responsible for the out of pocket expense.
Documents:
CLICK BELOW
Yellow Fever Vaccine InformationTravel Itinerary
Prescribing Information
Recommendations:
The yellow fever vaccine MUST be given 10 days before departure. However, it is recommended that the vaccine be given at least 30 days before leaving to a yellow fever endemic area. The vaccine appointment does not count as, or take the place of, a travel consult. The vaccine recipient should seek out a full and complete travel consult from their PCP or other qualified healthcare provider prior to departure.
What is Yellow Fever:
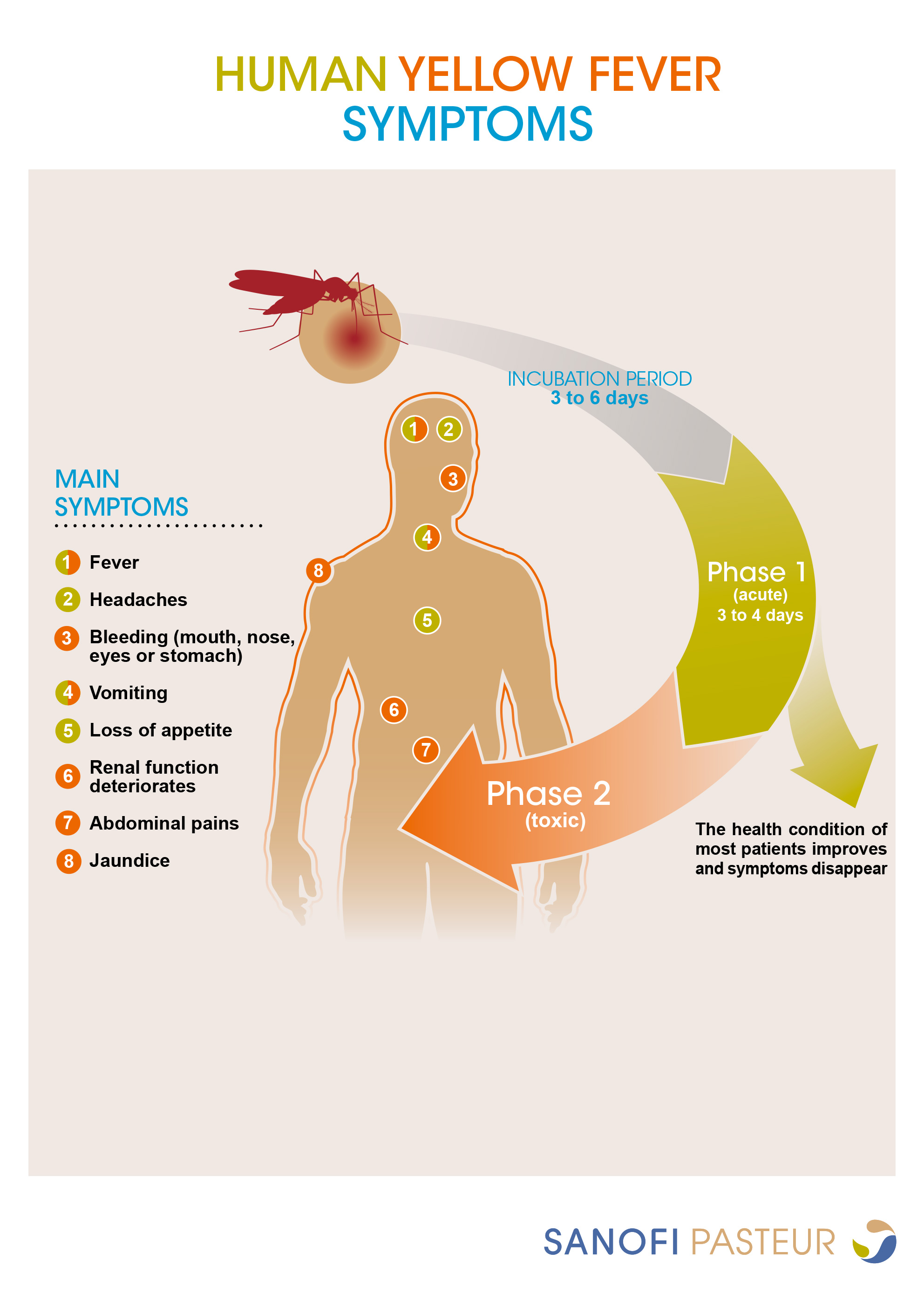
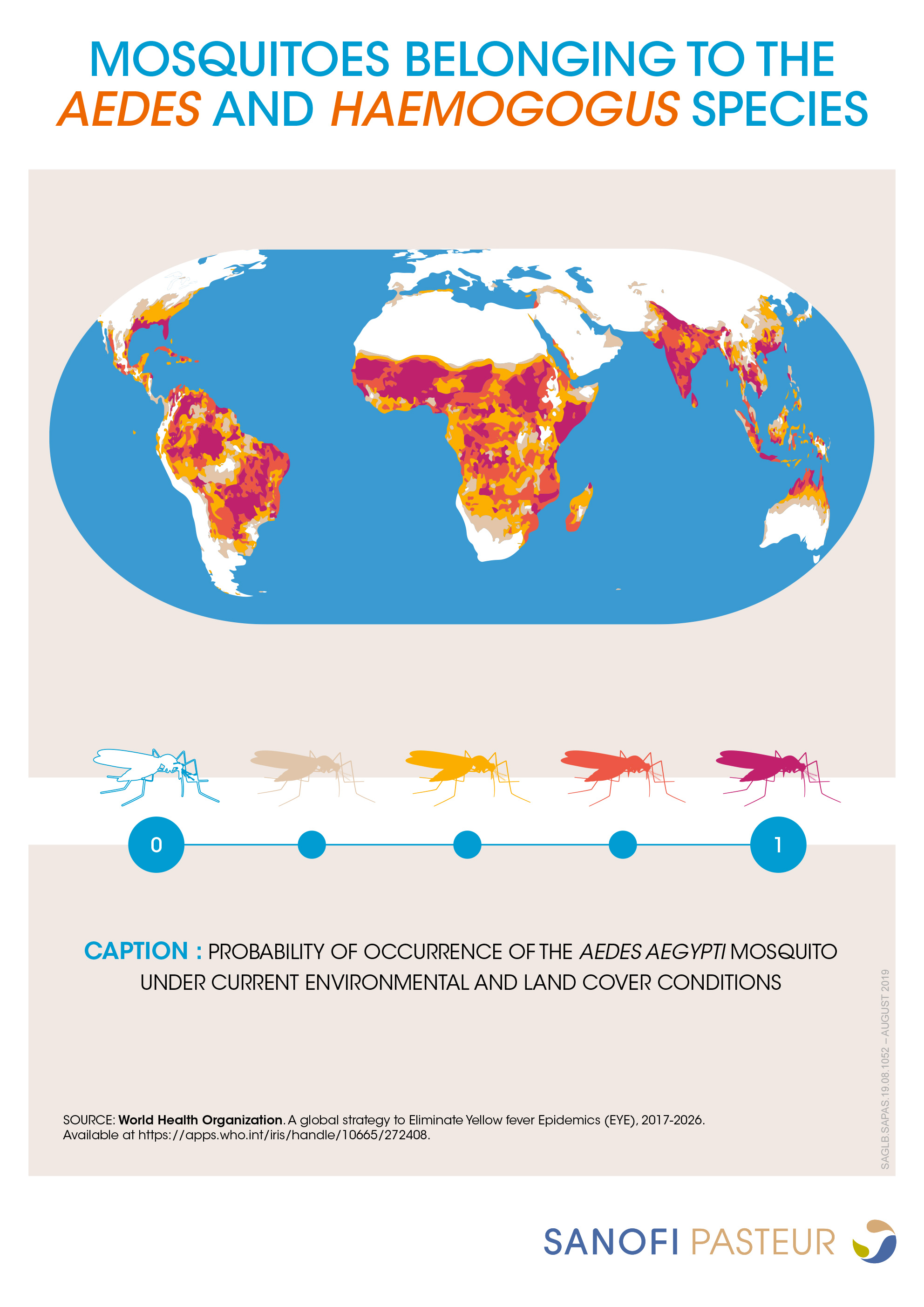
Yellow fever is endemic to parts of sub-Saharan Africa and tropical Central and South America and is spread by infected mosquitoes. Onset of yellow fever is typically abrupt, and progression of the disease occurs in several phases following an incubation period of about 3 to 6 days in which the patient may be asymptomatic or experience non-specific flu-like symptoms. The acute phase, phase 1, consists of symptoms such as fever, headaches, vomiting, or loss of appetite. Many patients recover completely at this stage after about 3 to 4 days. However, in about 5 to 26% of cases, patients progress to the toxic phase, phase 2, a more severe form of the disease that presents with epigastric pain, liver failure, renal failure, jaundice, and hemorrhagic (bleeding) complications. In those with severe disease, case-fatality ratios range between 30 to 60% with death occurring approximately 7 to 10 days after entering the second phase.
A safe and effective yellow fever vaccine has been available since the 1930’s. The yellow fever vaccine approved for use in the United States, called YF-VAX, is a highly effective, live attenuated vaccine developed by Sanofi Pasteur. The vaccine contains the 17D strain of the yellow fever virus which protects against all strains of the virus that are circulating in nature. YF-VAX is recommended for those, with no contraindications, who are travelling to areas where yellow fever is present or are entering a country where vaccination is a legal requirement. Upon receiving your yellow fever vaccine, you will be given an International Certificate of Vaccination or Prophylaxis (ICVP), also called the “yellow card,” that documents your vaccination. We recommend that you keep your ICVP readily retrievable with your passport as you will be required to present proof of vaccination upon entry into certain countries. The vaccine must be administered at least 10 days before travel to a yellow fever endemic region to allow for protective antibodies to develop. However, it is highly recommended to receive the vaccine at least 30 days or more before departure to allow neutralizing antibodies to develop as well.
Mild adverse events occur in 5 to 30% of patients who receive the yellow fever vaccine. Common side effects of the vaccine are mild and include fever, headache, backache, myalgias, or injection site inflammation. Myalgias, or muscle aches, can start within several days of vaccination and last 5 to 10 days. Injection site inflammation can start anywhere from 1 to 5 days following the injection.
More serious adverse events can occur but are extremely rare. This includes hypersensitivity reactions, neurological disease (encephalitis) and viscerotropic disease (damage to the kidneys, liver, and lungs). The yellow fever vaccine can cause anaphylaxis on rare occasions, with a rate of 1.3 cases in 100,000 doses. Less severe hypersensitivity reactions can also occur, such as rash or urticaria. Those with a history of hypersensitivity to eggs, egg products, chicken protein, or gelatin are contraindicated from receiving the vaccine. Yellow fever vaccine-associated neurological disease (YEL-AND) occurs in approximately 0.8 out 100,000 doses received and is serious but rarely fatal with most patients recovering completely. It primarily presents as encephalitis (inflammation of the brain) that can progress to nervous system involvement. Yellow fever vaccine-associated viscerotropic disease (YEL-AVD) mimics the signs and symptoms of yellow fever disease and can cause multiple organ disfunction and failure. It is rare but serious and occurs in approximately 0.3 out of 100,000 doses administered. Infants less than 9 months old and adults 60 years or older are more likely to experience YEL-AND and YEL-AVD. If any severe side effect develops following your vaccination, consult a physician and notify the vaccinating clinic.
The yellow fever vaccine currently in use is an extremely effective and safe vaccine that has been available for several decades. A single dose of the vaccine is sufficient for most individuals to develop a lifetime protection and sustained immunity against the virus. In very rare cases, a booster dose may be recommended. The vaccine provides 80% of patients with effective immunity within 10 days of vaccination. Within 30 days of vaccination, more than 99% of patients will develop effective immunity against the virus. The yellow fever vaccine is the single most important step in preventing yellow fever.
Sources:
Centers for Disease Control and Prevention. Yellow Fever Vaccine (Rev 2019): Information for Health Care Professionals Advising Travelers. Updated September 3, 2019. Accessed January 21, 2023 https://www.train.org/cdctrain/course/1079977/compilation
Sanofi Pasteur. Human Yellow Fever symptoms. Flickr. https://www.flickr.com/photos/sanofi-pasteur/8599167981/in/album-72157686673590684/. Published February 27, 2013. Accessed February 22, 2023.
Centers for Disease Control and Prevention. Chapter 2: Preparing International Travelers. CDC Yellow Book 2020: Health Information for International Travelers. New York: Oxford University Press; 2017.
World Health Organization. Yellow fever. Updated May 7, 2019. Accessed March 15, 2023. https://www.who.int/news-room/fact-sheets/detail/yellow-fever
Gotuzzo E, Yactayo S, Córdova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89(3):434-444. doi:10.4269/ajtmh.13-0264
Staples JE, Bocchini JA Jr, Rubin L, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow Fever Vaccine Booster Doses: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(23):647-650.
Useful Links for Travel Information - Patients:
https://wwwnc.cdc.gov/travel/destinations/list
CDC Traveler Advicehttps://wwwnc.cdc.gov/travel/page/traveler-information-center
CDC Pre-Travel Quick Guidehttps://wwwnc.cdc.gov/travel/page/pre-travel-guide
CDC Yellow Bookhttps://wwwnc.cdc.gov/travel/page/yellowbook-home-2020
CDC Travel Noticeshttps://wwwnc.cdc.gov/travel/notices
Travel Noticeshttps://travel.state.gov/content/travel/en/traveladvisories/traveladvisories.html/
Find the Repellent that is Right for You – EPA (includes a search tool)https://www.epa.gov/insect-repellents/find-repellent-right-you
Repellents: Protection against Mosquitoes, Ticks, and Other Arthropods - EPAhttps://www.epa.gov/insect-repellents
Choosing and Using Insect Repellents (Includes extensive information and links regarding children and use during pregnancy and nursing)http://npic.orst.edu/ingred/ptype/repel.html
Insect Repellents Fact Sheet – NPIChttp://npic.orst.edu/factsheets/repellents.html
Insect Repellents and Children – AAPhttps://www.healthychildren.org/English/safety-prevention/at-play/Pages/Insect-Repellents.aspx
Preventing Mosquito Bites – CDChttps://www.epa.gov/insect-repellents/tips-prevent-mosquito-bites
Photo Gallery:
Yellow Fever FAQs
The Short Answer: Most individuals who have previously received a yellow fever vaccine do not need a booster dose even if their ICVP states that they are due for one. The ICVP should be accepted for the lifetime of the patient vaccinated under the new rules from the International Health Regulations which became effective in 2016. The addition to the IHR states that “the amendment provides that the period of protection from vaccination with an approved vaccine against infection with Yellow Fever, and the validity of the related certificate, will be for the life of the person vaccinated rather than a period of ten years as previously required.”
The Long Answer: Some individuals may require a booster dose if they meet certain criteria. Those who had their vaccine more than 10 years ago and will be traveling to a location that has reported an active and ongoing outbreak can choose to get a booster dose. Additionally, those who were pregnant at the time of their last vaccination, have received a hematopoietic stem cell transplant in the time since their last dose, or are HIV positive may be eligible for a booster dose. Travelers should also check if the country they are traveling to requires a booster dose in the case that the country has not yet adopted the new IHR amendment.
If your need a new ICVP and you received your dose through the clinic at Boone Drug at Deerfield, you can call our store and request a new card. We will need you to drop by the clinic with your most up to date passport so we can issue you a new card. If you are unable to travel to the clinic due to distance or other circumstances, we would be happy to discuss with you alternative ways of getting you a new card.
If you received your yellow fever vaccine at a clinic that is not Boone Drug at Deerfield, you will need to contact that clinic for a new copy of your ICVP.
Reactions to the vaccine are generally mild and include headaches, muscle aches, and/or low-grade fevers. Severe and serious side effects can occur but are extremely rare.
The yellow fever vaccine, YF-VAX, is a live attenuated vaccine.
The yellow fever vaccine, YF-VAX, has components that could potentially cause a hypersensitivity reaction in select individuals. These components include gelatin, eggs, egg products, or chicken protein. If you have a history of hypersensitivity reactions to any of these components, this is a contraindication to receiving the vaccine.
If you have recently received the MMR vaccine or other live vaccinations, you should separate your yellow fever vaccination by 30 days from the date the other live vaccine(s) were given. However, you can receive the MMR and the yellow fever vaccine on the same day.
The oral typhoid vaccine is a live vaccine but can be given at any interval relative to the yellow fever vaccine. Inactivated vaccines do not interfere with administration of the yellow fever vaccine.
Your dose of yellow fever vaccine is valid 10 days after it is administered, so receiving the vaccine at least 10 days before your departure date is required. However, we recommend receiving your dose at least 30 days prior to your departure date.
Your ICVP must list your passport number, your name and additional information exactly as it is listed on your passport. Our clinic staff will need to reference your passport when completing your ICVP.